URBANA – Researchers at the Cancer Center at Illinois have developed a new technique aimed at improving the way cancer diagnoses are performed.
The approach combines an infrared laser, a high-resolution optical microscope and machine-learning algorithms to generate high-resolution images paired with detailed chemical information about the cells within a patient’s tumor.
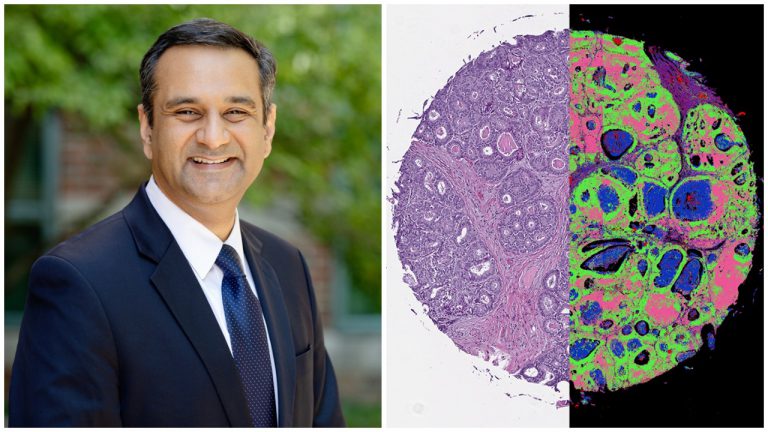
Typically, tissue biopsies involve using color dyes to identify cellular features within an ultra-thin slice of tissue removed from the patient’s body, said U of I bioengineering professor and Cancer Center Director Rohit Bhargava.
This approach to analyzing tissue biopsies works well for distinguishing between cancer cells and healthy cells, Bhargava said. But when it comes to determining the severity of disease, sometimes “if you give the same sample–particularly the difficult cases–to two different (clinical pathologists), you might get two different opinions. And that could change the way we treat the patient.”
So Bhargava’s research group set out to develop a new technique that could generate more data to help clinicians determine the best treatment for a person’s cancer.
The results are published in the Journal of the National Academy of Sciences.
Bhargava spoke with Illinois Newsroom about the approach.
This interview has been lightly edited and condensed for clarity.
Christine Herman: What are some of the limitations to the typical approach to cancer diagnosis, involving dyes and optical imaging?
Rohit Bhargava: There are a few limitations. It is a time consuming process. There are chemical reagents that need to be used, which in itself is not a limitation, but it does cost money and delay diagnosis a little bit. And finally, we have humans looking at things that could perhaps be helped by computer programs and artificial intelligence, providing some input so that we can make better decisions. So that opportunity doesn’t exist yet.
CH: Is it correct that sometimes with the traditional approach involving dyes and an optical microscope, it can be difficult to distinguish cancer cells from healthy cells? Or to pinpoint the boundaries of a tumor? Why is it important to be able to do that?
RB: That’s a great point. So the reason it’s really important is because it could determine the course of therapy. For example, looking at cells in the margins, and having clear margins, can assure us that surgery went well, and we don’t need to re-intervene in that patient, so we could prevent future procedures.
It’s not so much that there’s difficulty in distinguishing late stage cancers from normal tissue; that’s relatively well done. The trouble happens when there are very subtle changes that characterize the severity of the disease.
So for example, we don’t know if this is a particularly serious form, or it’s slightly less serious form. And sometimes because we’re only looking at shape, and we’re only looking at the appearance of a disease, which is actually inherently molecular, we make mistakes in how we look at it.
CH: I understand part of your research group’s motivation for this new study was to add to the toolkit that’s available to people who are trying to make accurate diagnosis of cancer. How did you set out to do that?
RB: So we’ve been working on this technique that we call chemical imaging for a while now, more than 10 years. And the idea here is to not just measure the shape that you see in a microscope, but measure the chemistry. So at every pixel, instead of getting a red, green and blue color, we get a list of chemical compounds and their concentrations.
Now suddenly, instead of just thinking of cancer as how it appears under a microscope slide, we can ask the question: What is it composed of? And the composition is really what drives cancers in different ways.
The problem was that you had to build whole new instruments that were not really compatible with traditional microscopes. So we had to acquire traditional images and then we had to acquire infrared images. And then we had to combine the data in some way. So this latest work from our lab actually combines all of that into one platform.
CH: So you’ve built this new hybrid microscope that kind of joins forces of the infrared analysis and the visual high-resolution imaging of the optical microscope?
RB: Absolutely.
CH: What does the machine-learning component involve?
RB: It’s not possible for humans to look at individual chemistry and the long list of chemicals that we can detect. It’s also not possible for humans to appreciate the very subtle structural changes, but machine-learning algorithms are very, very powerful. They’re very good when you can identify a task and say: Could we characterize a particular pattern either in space or in chemistry?
And so we are using machine learning algorithms to identify automatically the chemistry as well as the structure within tissues so that we can pinpoint cancer and other changes that accompany that.
CH: What needs to happen before this technique might become more widespread, or even replace some of those standard tissue staining techniques that are currently used in cancer diagnosis?
RB: So one of the really big advantages–a problem that this technique that we report here in this paper solves–is that it provides us the level of detail in space that a pathologist is used to seeing. So they see the same quality images.
But now they have much more information. So we’ve achieved the sort of technological limit of providing the same information and then adding some more details to it. We’ve also, in other words, shown that using machine-learning algorithms, we can not only reproduce what we can do in the clinic, but perhaps exceed in terms of quality and understanding disease.
Now what needs to happen is we need to take a solution and apply to different problems so that we can validate this on large numbers of patients and then we’re ready for this to be introduced in routine practice.
Follow Christine on Twitter: @CTHerman